While we’re still waiting for the FDA to release guidelines on CBD, there are a handful of FDA-registered topical products with CBD in them. In this episode of the Ministry of Hemp podcast, we learn a bit more about one and the process of getting registered.
First off, our podcast host Matt talks about the difficulties of reading and understanding CBD labels. The good news is MOH’s new videographer, Desiree Kane, just dropped a fantastic short video about How to Read CBD labels.
This episode’s conversation is with Ed Donnelly of AmourCBD. Ed has 35-years of experience in healthcare as everything from a burn unit nurse to a CEO. Donnelly got into the CBD business after his wife was injured and found pain relief in topical CBD but it was stinky and slimy. So Ed decided to start his own line of CBD for pain relief and make a better FDA registered product.
Send us your hemp questions and you might hear them answered on one of our Hemp Q&A episodes. Send your written questions to us on Twitter, Facebook, [email protected], or call us and leave a message at 402-819-6417. Keep in mind, this phone number is for hemp questions only and any other inquiries for the Ministry of Hemp should be sent to [email protected]
Be sure to subscribe to the Ministry of Hemp podcast on Spotify, Apple Podcasts, Podbay, Stitcher, Pocketcasts, Google Play or your favorite podcast app. If you like what your hear leave us a review or star rating. It’s a quick and easy way to help get this show to others looking for Hemp information and please, share this episode on your own social media!
If you believe hemp can change the world then help us spread the word! Become a Ministry of Hemp Insider when you donate any amount on our Patreon page. You’ll be the first to hear about everything going on with our special newsletter plus exclusive Patron content including blogs, podcast extras and more. Visit the Ministry of Hemp on Patreon and become an Insider now!
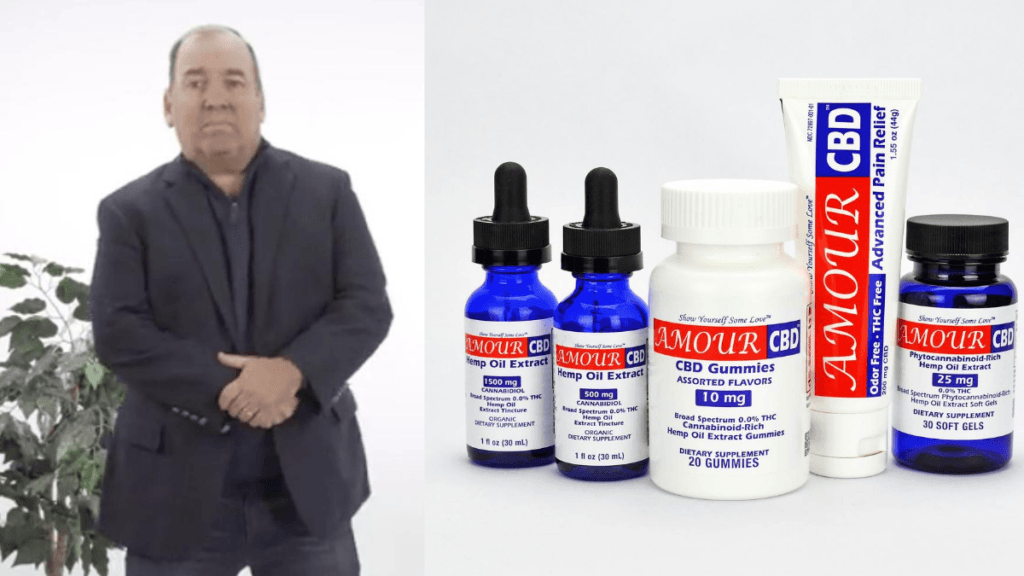 After his wife injured her back, Ed Donnelly created Amour CBD, makers of an FDA-registered topical product with lidocaine and CBD.
After his wife injured her back, Ed Donnelly created Amour CBD, makers of an FDA-registered topical product with lidocaine and CBD.
Below you’ll find the complete transcript of episode 49 of the Ministry of Hemp Podcast, “FDA registered topical CBD products”:
Matt Baum:
I’m Matt Baum, and this is the Ministry of Hemp podcast, brought to you by ministryofhemp.com, America’s leading advocate for hemp and hemp education.
Matt Baum:
Welcome back to the Ministry of Hemp podcast. My name is Matt Baum. I’m your host, and today on the show, we are going to talk to Ed Donnelley of Amor CBD. Now, I apologize for this. When I talked to Ed months ago, but there was some audio problems and I want to thank my friend, Ben, who helped me out with this one and rescued the audio. So we’ll get to that in a little bit. But before we do talk to Ed, we should talk about labels for a minute. Because there is no current FDA rules on CBD, there’s no hard and fast rules on what the labels need to say to let you know what you’re getting, and that can be very confusing.
Matt Baum:
I just got sent a sample today. It’s a CBD tincture that’s supposed to help with sleep, and it says it’s 25 milligrams per dose, which that’s very cool. I like that. As opposed to a label that just says, here’s 1500 milligrams of CBD or here’s 200 milligrams. Well, that’s tough when it comes down to how much is the dosage? It’s hard to read. It’s hard to know what you’re getting. Like I said, there’s no hard and fast rule from the government that says, you need to make it say this so people know exactly what they’re getting. Now we’re moving towards that. It’s going to happen. But for now CBD labels can be a little tough to read. Fear not, we have a new videographer. She’s awesome. Her name is Desiree Kane, and she just put out a video on how to read CBD labels.
Matt Baum:
She talks about what to look for, what to avoid, and all kinds of other stuff in a new video we’ve done up on ministryofhemp.com. I’ll have a link to it in the show notes for this episode, but you’ll be able to find it featured on ministryofcom.com right now. Desiree is awesome. We’re thrilled to have her as a part of the team, and she does such a fantastic job just breaking down how to read different labels on different CBD products. She does a great job touching on a few of them and helps you to understand what they’re saying.
Matt Baum:
It sucks that we even have to make a video like this right now, but until the FDA rules exactly how these labels should look and what they should say, good to have someone like the Ministry of Hemp and Desiree to help you navigate the wilds of these different CBD labels.
Matt Baum:
My conversation today is with Ed Donnelly. He’s the founder and CEO of Amor CBD. Ed worked in healthcare for 35 years. He’s been the CEO of three different related companies as well. He was a registered nurse, worked in a burn unit for five years. Ed first discovered CBD not too long ago when his wife was injured and he decided that he wanted to make the product even better. I spoke to Ed in his home city of Chicago. Here is my conversation with Ed Donnelly.
Matt Baum:
Ed, so your story starts back on September 11th, 2001, if I’m correct?
Ed Donnelly:
There is a story that starts there, yeah. No, that was a bad day. My brother was a fireman. He was with Ladder Company 3, 20, 21 years on the job, and he got killed in the Trade Center.
Matt Baum:
Wow.
Ed Donnelly:
So if you go to the museum, the ladder truck that’s crushed in the center of the museum?
Matt Baum:
I’ve been there.
Ed Donnelly:
[inaudible 00:03:53] truck. That’s his, and he was the senior officer.
Matt Baum:
Wow. Wow. That is …
Ed Donnelly:
It was a Green Beret company.
Matt Baum:
They were all military guys is what you’re saying?
Ed Donnelly:
Yeah. This was a heroic group of guys. My brother had three department medals.
Matt Baum:
Wow.
Ed Donnelly:
Not unit citations, three personal medals. They give out 12 a year, and he had three.
Matt Baum:
You couldn’t have kept these guys out of there if you wanted to.
Ed Donnelly:
Absolutely. Then my brother, we got a report that he was on the 65th floor of the North tower and a woman coming down offered him a Diet Coke, and he smiled at her and said, “That would just be a tease, dear.” She remembered his smile, but absolutely he was going up to the floor.
Matt Baum:
Wow. After that, you basically create your own CBD company.
Ed Donnelly:
I’ve been in healthcare for 35 years, the last 25 as CEO of three different companies, one being a public company. So, 2017, my wife takes a fall, hurts her back, doesn’t want opioids, so I go out and I find CBD and it works tremendous for her, but the problem is, it had a terrible odor, the cream, and it contained THC. So I looked at this and, again, with my background as a medical company CEO with FDA experience, I said, this could be done better.
Ed Donnelly:
So I went out and did Amour. It’s odorless, it has zero THC, and it’s not greasy. So that was our pain relieving cream, and as I dove into it, I realized this needs to be registered with the FDA.
Matt Baum:
Absolutely.
Ed Donnelly:
But nobody’s doing it.
Matt Baum:
That’s the biggest problem we’re facing right now.
Ed Donnelly:
Nobody’s doing it. So I took a year and spent a lot of money and a lot of time to get Amour CBD cream FDA registered, which in my view, it’s not FDA approved-
Matt Baum:
It’s registered.
Ed Donnelly:
It’s registered. It gives people the Good Housekeeping Seal of Approval that they know that this is a safe product manufactured to standards. Because you can buy CBD today in a gas station, and you don’t know if that CBD oil is coming from China, from Russia, they have THC, they have solvents in them.
Matt Baum:
Or do they even have CBD in them at all? You have no clue.
Ed Donnelly:
100% right. We do all the independent third party testing, and I told somebody in one other interview one time, my goal, I guess, was to do CBD right. I was very successful in my career as public company CEO, and if I’m going to do it and put my name to it and we have no outside funding, I’m funding it all, I’m going to do it right.
Matt Baum:
Tell me real quick about FDA registration. So this is not approval. This is registration. That basically means, what kind of hoops did you jump through? You said, you laid out this is how we will create it. This is how it’s made. This is what goes into it. Is it stuff like that?
Ed Donnelly:
Yeah. If you go to Walgreens, CVS, wherever, Target, and buy cough syrup, buy Tylenol Cold and Flu, whatever you buy, there is an NDC number, National Drug Code, and it’s given a number, meaning that they’ve gone through the FDA registration process. You’ve told them what’s in it. You’ve done an independent analysis. You’ve told them that it’s being manufactured in an FDA certified facility, not in the back of some guy’s garage or basement. So you go through all these FDA good manufacturing standards to prove that the product is what you say it is.
Matt Baum:
Fair enough.
Ed Donnelly:
Then they approve the labeling, they approve the claims, which generally there are no claims. We’re able to say advanced pain relief, but you can’t say that it cures cancer.
Matt Baum:
Of course, which is good. We shouldn’t be.
Ed Donnelly:
We shouldn’t. Absolutely, we shouldn’t.
Matt Baum:
Correct me if I’m wrong. But your cream includes lidocaine, which is typically used for burn victims. This comes from your background working with burn victims. Is this correct?
Ed Donnelly:
That is correct. As scary as it seems, I’m a registered nurse. I had training. You wouldn’t want to get sick in front of me, but I am a registered nurse. I’m clinically trained and I did work in burn unit for five years. Lidocaine is using in burn centers, but lidocaine is used every place. Novocaine in the dentist office is a form of lidocaine. Lidocaine is absolutely the number one topical pain reliever on the market. Lidocaine. It’s in Neosporin. They sell Neosporin with lidocaine. So it’s, without a doubt, the best pain reliever. But yes, I’m a nurse in my undergrad. It’s very important to me.
Matt Baum:
Absolutely.
Ed Donnelly:
It’s who I am. I’ve got a patient sensitivity.
Matt Baum:
Absolutely. So you took your nursing undergrad and from there, you went straight into opening businesses? Or you said, “You know what, I’m tired of this nursing thing. I need to make some money?”
Ed Donnelly:
I was working as a nurse in a burn unit, working all the overtime I could get because back in 1980, nurses didn’t pay that great. But I went to business school at the same time. So I was taking care of the sickest people in the burn unit with the curtains drawn and read some books there. I was reading and I was typically one to one patient care. Got out of a business school. I worked as a nurse for five years, so it’s not like I just did it part time. Then I went and worked for big Fortune 100 medical companies, so I didn’t just go out and start businesses. I went and started as a sales rep, ran a region, moved to Dallas, ran half the country, moved to the headquarters, ran the whole country, big company, and then recruited away to be a president of a good sized company, which I made a big company. There’s another one that I took public on the NASDAQ.
Matt Baum:
Tell me how you came to find CBD and hemp. You said your wife had an accident.
Ed Donnelly:
My wife fell. My wife fell and hurt her back and she had chronic back issues to begin with. So again, she didn’t want opioids. Again, dangerous nurse medical guy. I’ll go find something, and I found CBD. Now, we put this greasy, smelly stuff on her, but it worked. It absolutely worked for her.
Matt Baum:
It just didn’t smell good.
Ed Donnelly:
We also had her taking some drops too. She would take the drops under her tongue and maybe even chew some gummies. Suddenly she was getting comfort and relief, not a cure, but able to get through her day. Then I said, “This could be done better.” These products, thank you for being there, but they’re not as good as they could or should be. The THC. There are horrible stories here in Chicago about a bus driver taking CBD, takes a urine test and fails it.
Matt Baum:
Then blacklisted forever.
Ed Donnelly:
Yeah, and then she got fired. Terrible.
Matt Baum:
It’s just ridiculous.
Ed Donnelly:
There’s no reason to have the THC. It takes another couple hours of processing per tube to get rid of the THC. So in some ways, like everything else, it’s all about money. Guy wants to be selling it cheap in a gas station, so he’s giving you China grown marijuana with THC, might not be tested, but they get it out there and they put it out for 9.99. CBD, it’s the old story. You get what you pay for it.
Matt Baum:
Absolutely. Tell me about the hemp that you’re using.
Ed Donnelly:
Ours is US grown only. Colorado grown, but it’s absolutely US grown. We use independent third party labs-
Matt Baum:
Nice. That’s important.
Ed Donnelly:
… To test for THC and everything. So we’ve got all of the necessary proofs to demonstrate exactly what we say. Then again with the FDA, you darn well better do what you say. Otherwise, they’re going to be ringing your doorbell.
Matt Baum:
Was that difficult when you came to the FDA and you said, “Look, I want to register.” I would guess their first reaction would be to say, ‘No, we don’t deal in that.” What was that conversation like?
Ed Donnelly:
It’s interesting. It’s a great question. The conversation was easy because the FDA registration part is not difficult. It’s laborious and time consuming and expensive. Everything you’d expect from a government process.
Matt Baum:
Of course. Yeah. It wouldn’t be a government process otherwise.
Ed Donnelly:
That’s right. Fill out this long form and then take close to a year, submit multiple drafts. So that wasn’t hard. What was interesting was then going to the trademark office and trying to trademark Amour CBD. The government trademark office wouldn’t even listen to it because CBD is illegal, they say.
Matt Baum:
You may as well trademark heroin! What, are you crazy?
Ed Donnelly:
Yeah. But the FDA process wasn’t difficult. It was just it’s normal, tell us what you have. We have a pain relieving cream. Tell us what’s in it. Lidocaine and CBD. Okay, lidocaine, it’s a regulated drug, so it needs to be registered. So it was all just checking the boxes and taking the time, being patient and paying fees.
Matt Baum:
Fair enough. So as long as you are-
Ed Donnelly:
We’re the only one that does it. We’re the only one that’s done it. Why? Because it takes nine to 12 months and tens of thousands of dollars to do it.
Matt Baum:
You’re the first person I’ve talked to that has done it. I didn’t even know it was possible before I spoke to you about this, honestly.
Ed Donnelly:
For me, it’s because, again, as a previous CEO of medical companies, I dealt with the FDA all the time. So maybe for me, it was easier to get done, because the first thing I did was go to a FDA lawyer who was an expert on this and paid his ridiculous fee. But I knew the straight path.
Matt Baum:
This is all part of the game, right?
Ed Donnelly:
It’s a game of sorts. Absolutely.
Matt Baum:
Yeah.
Ed Donnelly:
It’s just checking the box and doing it right. It’s funny. Before this pandemic, I had interviews going where people asked me to make predictions for 2020 and what CBD was going to be all about, and every other person who was interviewed said it’s going to be a $20 billion business.
Matt Baum:
It’s going to be huge.
Ed Donnelly:
[crosstalk 00:16:11] billion-
Matt Baum:
Farmers are going to be millionaires!
Ed Donnelly:
All I said to the interviewer, because I’m sort of a regular guy. I said, “It’s going to be big. But the news flash for 2020 is, watch the FDA get involved.” Because the end of last year was when Juul and the vaping companies had already issues of people dying of respiratory problems and they tied it all back to THC.
Matt Baum:
Right, and that it’s the same thing as CBD, right? Must be the same. I guess that’s [crosstalk 00:16:47] people too.
Ed Donnelly:
Absolutely. THC is THC. They were just smoking it and ingesting it. That was why, when that happened, I was sickened for the individuals. I wasn’t happy because it cast a shadow on the CBD climate.
Matt Baum:
Yeah. It was really too bad.
Ed Donnelly:
But I was very happy that I had the foresight to go no THC in any of my products, either the cream, the liquids, the gels, or the pills. No THC whatsoever.
Matt Baum:
So let’s say, tomorrow the FDA, best case scenario of course, tomorrow the FDA says, “All right, we’ve got rules in place. We get it, and full spectrum is fine. If you want to have THC, it has to be below whatever, but it’s fine.”, are you going to switch or are you going to stay the way you are?
Ed Donnelly:
I’m going to stay the way I am. I’m going to say we’re broad spectrum, and I’m also going to take the position, which is the one I’ve been taking, is we’re a medical grade. We are a medical CBD. We’re not recreational. If people want the best, if they’re having difficulty with chronic pain in their shoulder, knee, I use it on my rotator cuff, it’s great. If people are having anxiety related issues due to COVID, they take the drops. I’m making sure my wife takes them twice a day.
Matt Baum:
Fair enough.
Ed Donnelly:
Because she’s stir crazy with this. I’m not going to change. We’re doing broad spectrum, no THC, medical grade. We’re not trying to compete with the 9.99. We’re putting out the best, safest, FDA product.
Matt Baum:
It’s sounds to me, the way that you’re talking. And I’m not demonizing anyone, I don’t think, but it sounds to me like you see your products in a pharmacy, not so much in a specialty CBD shop.
Ed Donnelly:
I think that’s a fair description with one caveat. I think we’re really looking for that educated consumer that does their homework, because our website is our number one source of getting it, amourCBD.com. But pharmacies are absolutely carrying our products.
Matt Baum:
Oh really?
Ed Donnelly:
Pharmacies, pro shops in the golf courses. They carry it because they know it’s the high quality. But certainly we’re not looking for that person who’s just experimenting with trying to be recreational with gummies. We have gummies, they’re broad spectrum and they’re more expensive than the other gummies.
Matt Baum:
Right now, you do the drops, you do the cream, and you do the gummies. What’s next for Amour? Where are you guys going?
Ed Donnelly:
And pills.
Matt Baum:
Pardon me, and the pills, capsules.
Ed Donnelly:
We do pills for those that don’t like it. I can see it evolving into patches.
Matt Baum:
Oh really?
Ed Donnelly:
Pain relieving. You can embed it in a patch. Right now, we’re not going there, but there are many other personal products that can have CBD infused in them. So right now, we’re trying to stay that high road of medical grade. I think we might find ourselves taking the cream into different sizes. So this is 1.5 ounces. We might go to seven or eight ounces or we might go down to one ounce.
Matt Baum:
That cream that you’re holding up, let me stop you real quick. How many milligrams of CBD are in there? What’s the dosage like?
Ed Donnelly:
It’s 1.5 ounces of cream, and there’s 200 milligrams of CBD.
Matt Baum:
That’s a good amount.
Ed Donnelly:
Yeah. We don’t cut corners. It’s not placebo. It’s a good amount. Then our pills, we’ve got them in 500 and 1500 milligrams. So again, those are the most popular. My son has a business, he’s been selling competitive CBD products and those were the most popular sizes. Then the gummies, the gummies are the gummies. 10 milligrams in a gummie.
Matt Baum:
Right. You take a couple of them when you need them and you’re good.
Ed Donnelly:
Some people say they help with the putting.
Matt Baum:
Fair enough. Yeah, I can see that. So I’m not going to ask you to speculate here, but you are a guy that’s been in the business for awhile, and I think you’ve got some insight that a lot of people working in CBD right now might not have coming from the drug world. We’re in a very weird place with the USDA and the FDA right now. How do you see this shaking out as far as government action, national action? Right now, there’s a bunch of different statewide regulations that are making it very difficult for people in other states everywhere because your labels got to look like this in one state, and it’s got to say this in a different state and it can only have this in one state. How do you see this shaking out, and how long do you think it’s going to take before we have real federal guidelines behind this?
Ed Donnelly:
When the vaping crisis took place in, what was that? Fourth quarter of last year, October, November, December, when that horrific thing was going on, I was fully expecting the FDA to come cracking down. In fact, I had heard that some of the bigger companies had gotten FDA letters.
Matt Baum:
That was the rumor.
Ed Donnelly:
[crosstalk 00:22:27] they’ll publish that there were letters, cease and desist, et cetera. So I expected 2020 to be the year of the FDA and I was going to sit back and enjoy it because we had done the work. I expected it to be very beneficial to my business. Then the pandemic hit and the FDA went completely a whole different direction.
Ed Donnelly:
I don’t think the FDA is at all focused on CBD now, and I think some of the bad behavior is going to be allowed to continue probably for this full 2020, if not most of 2021. I think they’re just elsewhere directed. Will they get back on it? Probably. They should. God forbid, there’s an incident like THC, where somebody gets injured or hurt. I don’t want to see that. But I think unfortunately the FDA’s eyes are off the ball. They’re elsewhere directly now.
Matt Baum:
If you have to guess, how long do you think we have? Just shot in the dark. No one’s going to hold you to this.
Ed Donnelly:
End of 2021, second half of 2021 or 2022, unless something happens.
Matt Baum:
That’s a ways off too. But guys like you, it sounds like you have taken the steps you need to, to get ahead of the curve by making sure you’ve stood in line and you paid the money and you showed them I’m doing it the way you want it done. I’m putting this in it.
Ed Donnelly:
It would have been happening now, if not for this whole COVID issue.
Matt Baum:
It sure does seem like that.
Ed Donnelly:
Yeah. Because they were like … You obviously were aware that some companies had gotten letters and there were things happening, but now that’s not the priority.
Matt Baum:
Yeah. Ed, before I let you go, can I ask, where does the name Amour come from?
Ed Donnelly:
The name Amour came from my 10 year old granddaughter who had two heart transplants.
Matt Baum:
Oh my God.
Ed Donnelly:
She’s 10 years old, and she’s had two heart transplants, the most recent one this past Christmas. But I think of her all the time, every day, and Amour just came. That’s her.
Matt Baum:
That’s beautiful.
Ed Donnelly:
[crosstalk 00:25:02] her name, and that’s how I came up with the name Amour.
Matt Baum:
That’s beautiful. Is she doing okay?
Ed Donnelly:
She’s doing great.
Matt Baum:
That’s great. That’s really good news.
Ed Donnelly:
Doing great. She’s a miracle, but I’m not trying to … I’ve been very lucky in my life. I’m having fun with these products. We’re doing good, but I’m not trying to cheat, borrow, and steal. We’re making good medical grade products that are helping people. The FDA thing is a big deal.
Matt Baum:
Yeah. Now that I know that and when people start asking me about it, I’ll definitely push that. It’s just sounds like, even if you’re not approved, at least you have everything done. You’ve got 90% of what they need done, and then when they do say, “Okay, you can have approval,” you got 10% more work to go as opposed to 100.
Ed Donnelly:
Yeah. The guys that don’t have it, when the FDA gets annoyed, they crack down, they take you off market. I don’t know how CVS’ of the world and Walgreens can be selling these products that are not FDA registered.
Matt Baum:
I want to thank Ed for coming on the show. He was a lot of fun to talk to, and FDA registration was not something that I have heard of, but it sounds important and extremely beneficial. I would hope that more smaller CBD companies are looking into this, because FDA regulation is coming and it is going to affect every aspect of this industry. Just like I talked about earlier, from the labels all the way to how this stuff is made and what goes into it and what is allowed and what is not and what we can and can’t say that it does. That’s good, and people like Ed, it sounds like he’s ready. He’s done the hard work. He’s done the research. He’s putting out a quality product, and when the FDA does come knocking, he’s going to be ready for them. You’ll be able to find links to Amour CBD and all their products in the notes for this episode.
Matt Baum:
That brings us to the end of this episode, but there is still so much more Ministry of Hemp to keep you busy while you wait for the next one. Head over to ministryofhemp.com, and we have got some new CBD reviews from Well Care Botanicals Hemp about their new extract. It’s a high potency CBD without the hempy flavor if you don’t care for that earthy, hempy flavor. Personally, I like it, but some you don’t and that’s fine. Check us out on medium.com, at Ministry of Hemp, where you can find a new article we have about the science of mixing CBD and caffeine. Back in episode 43, I interviewed Alan Mortada of Ott Coffee out of Austin, Texas, and we both gushed about how much we like CBD and caffeine mixed together. Turns out it works pretty well, and there doesn’t seem to be many side effects.
Matt Baum:
If you need even more than that, follow us on all your favorite social media at Ministry of Hemp/ministry of hemp, and speaking of /ministry of hemp, if you go to Patreon/ministryofhemp, you can become a Ministry of Hemp insider, and directly help us to spread the good word of hemp to other people. Any amount you can give makes you a Ministry of Hemp insider, gets you access to podcast extras. We’ve got a great video up right now featuring the Harney brothers, who I interviewed a couple episodes ago, planting their own hemp. It’s actually pretty hilarious. You get early access to articles. You get Patreon exclusive articles and stuff too, but best of all, you’re helping us help spread the word of hemp. So if you care about this and you want to hear more, become a Ministry of Hemp insider. I can’t stress how cool it is and how much it really helps us.
Matt Baum:
Here at the Ministry of Hemp, we believe that an accessible world is a better world for everyone, so you can find a full written transcript of the show in the notes as well. It’s time for me to get out of here. I like to end the show the same way every time. I like to say, remember to take care of yourself, take care of others, and make good decisions, will you? This is Matt Baum with the Ministry of Hemp signing off.


Can CBD help with the anxiety and stress of being an out member of the LGBTQ community in a straight world? In this episode...

With hemp off to a shaky start in the U.S., we thought we’d take a look at Canadian hemp with help from an industry...

A new techology called a “cocrystal” could improve our ability to absorb CBD oil. Welcome to episode 62 of the Ministry of Hemp Podcast....